Evaporation Studies Using Stable Isotopes
Ongoing research has emphasized development and refinement of theoretical models describing isotopic behaviour at the air-water interface, including practical applications such as tracing of the evaporation process. A brief theoretical review is presented below.
Theoretical Background
The stable isotopes of oxygen () and hydrogen (
) are nearly ideal
tracers of water cycling processes as they are mass-conservative,
and occur naturally within the water molecule (i.e.
,
, and
). Systematic isotopic
labelling of water parcels within the hydrologic cycle (i.e.
atmospheric moisture, precipitation, surface water, groundwater
etc.) has been recognized globally, and is known to occur due to
isotopic fractionations which accompany phase transitions and
diffusive transport, owing mainly to slight differences in
molecular behaviour of the rare, heavy isotopic species. Such
passive labelling has been applied for example, to study mixing
between compartments in the hydrologic cycle during snowmelt and
runoff events. For open-water bodies, the isotopic fractionation
occurring during evaporation is most important, as it imparts a
distinct enrichment of heavy isotopes in lake water with respect
to inflow sources and precipitation.
For an evaporating water body, isotopic
fractionation occurs at the air-water interface due to slightly
lower vapour pressures and retarded rates of molecular diffusion
of the two rare, heavy isotopic species of water and
with respect to the
common, light isotopic species
. These fractionation processes, which typically give
rise to evaporating moisture which is isotopically depleted (lighter)
with respect to the residual liquid, have been exploited to study
evaporation by several approaches, including: (1) tracing of
isotopically-depleted evaporate admixtures to the atmosphere as
recorded in the deuterium excess of precipitation; (2) tracing of
vapour fluxes to the atmosphere and partitioning by measuring
isotopic gradients above the evaporating surface; and, (3)
tracing isotopic buildup which occurs in the residual liquid, i.e.
in lakes. Lake studies must apply an isotope mass balance
approach to account for exchange between lake water, inflow and
precipitation, and as a consequence have been a powerful tool for
study of water balance processes rather than evaporation alone.
An algebraic description of the fractionation process during
evaporation, and isotope mass balance is given below. A similar
description of isotopic fractionation accompanying freezing and
thawing is also presented and provides a basis for discussing the
significance of these processes in cold-regions lakes. A
representative cross-section of the broad spectrum of water
balance studies using isotopic methods is given elsewhere in this
volume.
Isotopic ratios are reported in standard "" notation as
deviations in per mil (‰) from the Vienna-SMOW (Standard
Mean Ocean Water), such that SAMPLE = 1000((RSAMPLE/RSMOW)-1),
where R is 18O/16O or
2H/1H. 18O and
2H values cited herein are normalized to -55.5‰
and -428‰, respectively, for SLAP (Standard Light Antarctic
Precipitation) (see Coplen, 1996). Analytical uncertainties are
±0.1 ‰ for d18O and ±2 ‰ for d2H.
Isotopic fractionation at the air-water
interface
Isotopic fractionation during evaporation can
be described as a combination of fractionations occuring by
exchange between water molecules in liquid and vapour (in
thermodynamic equilibrium), and diffusion of water molecules from
the liquid to vapour phase (also called kinetic or transport
fractionation). In the case of a water body in thermodynamic
equilibrium with the atmosphere i.e. when there is no humidity
gradient, the equilibrium fractionation factor (+) between
liquid and vapour can be represented as
(1)
where and
are the ratios of heavy isotopic species to the common isotopic
species in liquid and vapour, respectively where
, and
+ > 1
such that the rare, heavy isotopic species is more abundant in
the liquid phase. Isotopic differences between coexisting phases
are by convention discussed in terms of isotopic separation
factors. In this case, the equilibrium isotopic separation
between liquid and vapour (
) is given by
(2)
and and
are
the
-notation
equivalents of
and
, respectively.
Numerous laboratory measurements of have been conducted
for oxygen and hydrogen over wide range of temperatures such that
their values are reasonably constrained for use in water balance
studies (Fig. 1). Notably,
values are substantially better defined than the
analogous kinetic isotopic separations
(discussed later). For example, one
standard deviation for the results at 10°C is about 0.24 and 2.1
for oxygen and hydrogen, respectively, which is similar in
magnitude to the analytical uncertainty.
Figure 1. Variation in equilibrium isotopic separation
between liquid and vapour for oxygen () and hydrogen (
) as a function of temperature (°C).
Based on the data of: 1- Majoube (1971); 2-Bottinga and Craig (1969);
3-Jakli and Stachewski (1977), and; 4-Kakiuchi and Matsuo (1979).
The most commonly used values for oxygen and
hydrogen, valid for the temperatures of 0 to 100°C, are respectively (Majoube
1971):
(3)
(4)
which corresponds to an isotopic enrichment in and
, respectively, of
about 9.79 ‰ and 85 ‰ at 0°C and 11.71 ‰ and 112.3
‰ at 20°C, respectively. The ratio
varies over this temperature range between
8.68 to 9.59 and is similar to the slope of the Meteoric Water
Line (Craig 1961) in
versus
space.
For the case of evaporation into undersaturated
air, the isotopic separation between liquid and vapour typically
exceeds due to
kinetic effects. The total isotopic separation (
) in this case is
(5)
where is the kinetic isotopic separation which is
dependant on the evaporation mechanism. Craig and Gordon (1965)
first proposed the use of a Langmuir-type linear resistance model
to describe transport of the isotopic species through the
boundary layer during constant evaporation i.e. constant vertical
flux with no convergence or divergence in the air column. As
summarized recently in Gat (1996) the model depicts transport of
vapour through a series of sublayers, including in order: a
saturated sub-layer above the air/water interface, where relative
humidity
and the
isotopic ratio in the saturated sub-layer according to Eq. (1) is
; a boundary layer
consisting of a diffusive sublayer and turbulently mixed sublayer
in which transport occurs by diffusion and turbulent transfer,
respectively, and finally; the free-atmosphere where
,
and
being the specific
humidity in the saturated sublayer and the free atmosphere,
respectively.
Evaporation (through the boundary layer) of
water and the
rare, heavy isotopic species
(either
or
)
can be written, respectively as (Gat 1996):
(6)
(7)
where is the isotopic ratio in the free-atmosphere;
is the resistance to
transport of water through the boundary layer,
and
being the diffusive
and turburbulent contributions to
respectively; and
is the analogous resistance to transport
of the rare, isotopic species. Note that
as turbulent transfer does not cause
further isotopic fractionation and the resistance to transport of
the rare, heavy isotopic species
is dependent solely on the diffusive
resistance.
The isotopic composition of the evaporating
moisture flux (or evaporate), equal to the ratio of evaporation
of the rare isotopic species and water can then be determined by
(8)
expressed in notation where

(9)
where ,
,
and
are the
isotopic compositions of the evaporate, the liquid (assumed to be
well-mixed), and the free atmosphere, respectively.
For the case of resistance to mixing in the
liquid phase, Craig and Gordon (1965) propose
(10) 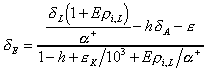
where is the resistance to mixing of the rare, heavy
isotopic species in the liquid phase. The effect of any
resistance, although widely presumed to be negligible, will cause
the evaporate to be slightly enriched relative to the liquid as
an enriched boundary layer will form in the near-surface liquid.
It is important to note that Eqs. (9) and (10) represent the net
evaporation process accounting for both evaporation and molecular
exchange (condensation) from the water surface to the free air.
According to the Craig and Gordon model, the
kinetic isotopic separation is defined as
(11)
The kinetic fractionation constant has been shown to be
related to the ratio of the resistance to transport of water
and the rare isotopic
species
through
the boundary layer such that
(12) 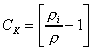
and
(13)
where and
are the molecular diffusivities in air of water and the the rare,
heavy isotopic species, respectively determined experimentally by
Merlivat (1978) and others, and
is a turbulence parameter. Experimental
evidence suggests that the turbulence parameter varies depending
on the evaporation mechanism such that
for mean turbulent flow,
for laminar flow and
for static transport (Gat
1996). This is consistent with a transient eddy model of a
randomly renewed surface layer as proposed by Brutsaert (1965,
1975). As noted in an earlier review (Gonfiantini 1986),
, which corresponds to
values of 14.3 ‰
and 12.5 ‰ for oxygen and hydrogen, respectively, appears to
reasonably represent open-water evaporation conditions (on the
time-scale of water balance investigations) that are most
frequently observed. In contrast,
is more appropriate for describing
evaporation from soils where static transport dominates.
The parameter , was recently introduced by Gat (1995)
to account for situations where the evaporation flux has a
significant influence on the free-air, as for the atmosphere on
the leeward side of large water bodies such as the Great Lakes of
North America (Gat et al.). In this situation precipitation The
adjusted humidity
,
in this case, is the humidity of the atmosphere following the
admixture of evaporate. In principle,
also can be used to describe any
situation where humidity and sampling of vapour for isotopic
analysis is conducted within the turbulently mixed sublayer.
A summary of the available estimates of the
kinetic fractionation constants is given in Fig. 2 based on
experimentally determined D/Di values for static transport (Merlivat
1978) at n=1, for mean turbulent (n=1/2) and laminar flow (n=2/3)
based on the model of Brutsaert (1965), and for transitional
regimes assuming continuity. Roughness reynolds numbers are also
shown for comparison.

Fig. 2 Roughness reynolds number zo+=u*zo/v where zo is
surface roughness length, u* is friction velocity, v is viscosity
of air.

Figure 3. Humidity dependence of the kinetic
fractionation eK for oxygen and hydrogen at the
air-water interface and its effect on the total isotopic
fractionation e , where e =
e*+ eK
, and e*
is the equilibrium fractionation and eK is the
kinetic fractionation (area between curves).
Isotope mass balance
For open-water periods when evaporation plays a
role in the water balance surface waters become enriched in the
heavy isotopic species. During such periods, the water-mass and
isotope-mass balance for a well-mixed reservoir, assuming
constant density of water, may be written respectively as
| (14)
|
||
| (15)
|
where V is the volume of the reservoir, t
is time, dV is the change in volume over time interval dt,
I is inflow, Q is outflow, E is evaporation,
and ,
,
and
are the isotopic
compositions of the reservoir, inflow, outflow, and evaporative
flux, respectively. Providing that isotopic compositions of
components in eq. (2) can be measured or estimated, and given
that systematic isotopic enrichment occurs during exposure to
evaporation under normal climate conditions, it is possible to
combine eq. (1) and eq. (2) to solve for two unknown water
balance components. While characterization of the isotopic
composition of most components is possible through weighted
sampling,
is difficult to measure
directly.
Constant Volume Models
Combining eqs. (2) and (3) and integrating
between the limits and
for time intervals where water balance fluxes
and their isotopic compositions can be assumed constant and
(i.e. hydrologic steady-state) yields
| (18)
|
where is the initial
isotopic composition of the reservoir, and
is the steady-state isotopic composition the
reservoir will attain as
determined by.
(19)
where is the fraction
of reservoir water lost by evaporation,
as defined in previous studies and
is the limiting isotopic composition under local climate
conditions.
In the special case where reservoirs are large
enough to buffer transient isotopic variations related to
seasonality of hydroclimate conditions, and when long time
intervals are considered, it can be assumed that the lake is also
close to isotopic steady-state () and eq.
(4) can be simplified to yield
|
(20) |
||
which is a key relationship describing the
dependency between the water balance of a large reservoir
and its isotopic enrichment by evaporation
. Atmospheric controls on this enrichment
include
and
through their influence on
and
.

Figure 4. Schematic time-series of d18O
enrichment in reservoirs for a range of x (evaporation/inflow).
Humidity effects are also shown.