424/525 In Detail
assignments | course materials | home | in detail | links | syllabus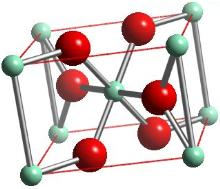
In detail
I plan to collect on this page some answers to questions I receive from 424/525 students. I expect them to largely be of the type that go slightly beyond or outside the course material, so this page is not examinable. However, you may find some of the answers will help you understand parts of the course better, and your questions will help shape future versions of the course. Questions may be submitted by email, but please be as specific as you can.
Q & A
Q8. When I work out the bond order for the Pt2 complex on the quadruple bonding handout (topic E), it comes out as a bond order of 2, but the table says it should be a bond order of 1. What's going on?
A8. This table was reproduced from p.1092 of Cotton and Wilkinson (5th edition), and it is in error.
The complex should have a 2- charge on it (p.1155, Greenwood and Earnshaw).
If you redo the calculation, it should come out correctly.
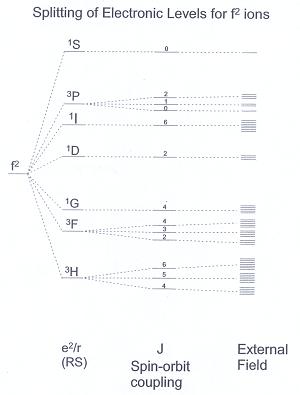
Q7. What sort of transition exactly gives rise to absorptions in the UV/Vis spectra of the Ln3+ ions?
A7. Electron-electron repulsion is significant for electrons in the compact f-orbitals and it is transitions between different arrangements of the fn configuration, e.g. for the f2 configuration, a transition between the ground 3H state and the excited 3F state.
The difference in energies between these states is of the order of 10,000s cm-1, and so are observed in the UV/Vis region. Transitions between different J states are much lower in energy (1000s cm-1) and the splitting due to the external ligand field is even smaller (~100 cm-1).
The external field splitting is important, however, as it relaxes the otherwise Laporte-forbidden f-f transition sufficiently that the absorption is observed, albeit weakly.
The weak vibronic coupling means that the absorptions are sharp (c.f. d-d transitions).
The figure below summarizes the relative importance of the various splittings.
Q6. Why are the values of the atomization enthalpies of the 3d metals anomalous in the centre of the row (especially for Mn)?
A6. Lower values often appear for metals that adopt a structure that is other than close-packed, and this is true for the 3d metals V to Cr (mostly bcc).
Mn has a particularly unusual cubic lattice in which there are four environments with coordination numbers of 12, 13 or 16.
Q5. How many coordination sites does the cyclopentadienyl ligand occupy in its various bonding modes?
A5. When eta5 (i.e. side-on), three (this stems from transition metal organometallic chemistry, where it consistently appears to occupy three sites in a fac arrangement).
eta2 and eta1 bonding modes each occupy one coordination site.
If you apply this reasoning to the three LnCp3 solid-state structures that were drawn in class, you will obtain coordination numbers of 10, 9 and 8.
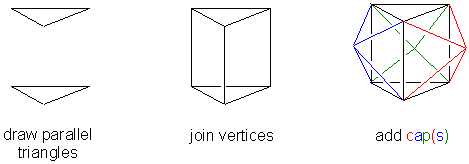
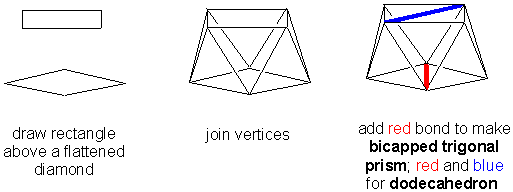
Q4. How do you draw the higher coordination numbers easily?
A4. Try these approaches:
(CAPPED) OCTAHEDRON

(CAPPED) TRIGONAL PRISM
SQUARE ANTIPRISM (AND RELATED)
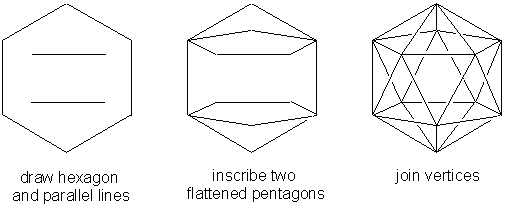
ICOSAHEDRON
Q3. When J = 0, gJ is undefined (e.g. for La, Lu). How do we calculate µeff?
A3. If J (total angular momentum) = 0, you can safely assume that the effective magnetic moment is 0 (a reasonable - and correct - conclusion, and it saves us having to worry about what 0 * 0/0 means...).
Q2. You said the third ionization energy was for the process [Xe] 4fn to 4fn-1, but what about La, Ce and Gd? The atoms have the electronic configuration [Xe] 6s2 5d1 4fn-1.
A2. True. However, once one or more electrons are removed, the order of orbital stabilities is not necessarily the same as in the undisturbed atom. The 4f orbitals are stabilised more by the loss of the 6s electrons than are the 5d orbitals, so the third ionization energy for La, Ce and Gd can still be thought of as the process [Xe] 4fn goes to 4fn-1.
Q1. Why do you use the term "lanthanides" when the periodic table you gave us calls these elements the "lanthanoids"?
A1. Lanthanoids is the IUPAC-recommended term (1971). However, it has never really caught on - the term lanthanides has been around a lot longer and has much wider usage (it's not always the best comparison, but try searching for the two terms on Google and comparing the number of hits).
© JS McIndoe, Department of Chemistry, University of Victoria.
