Hore group
research | group | papers
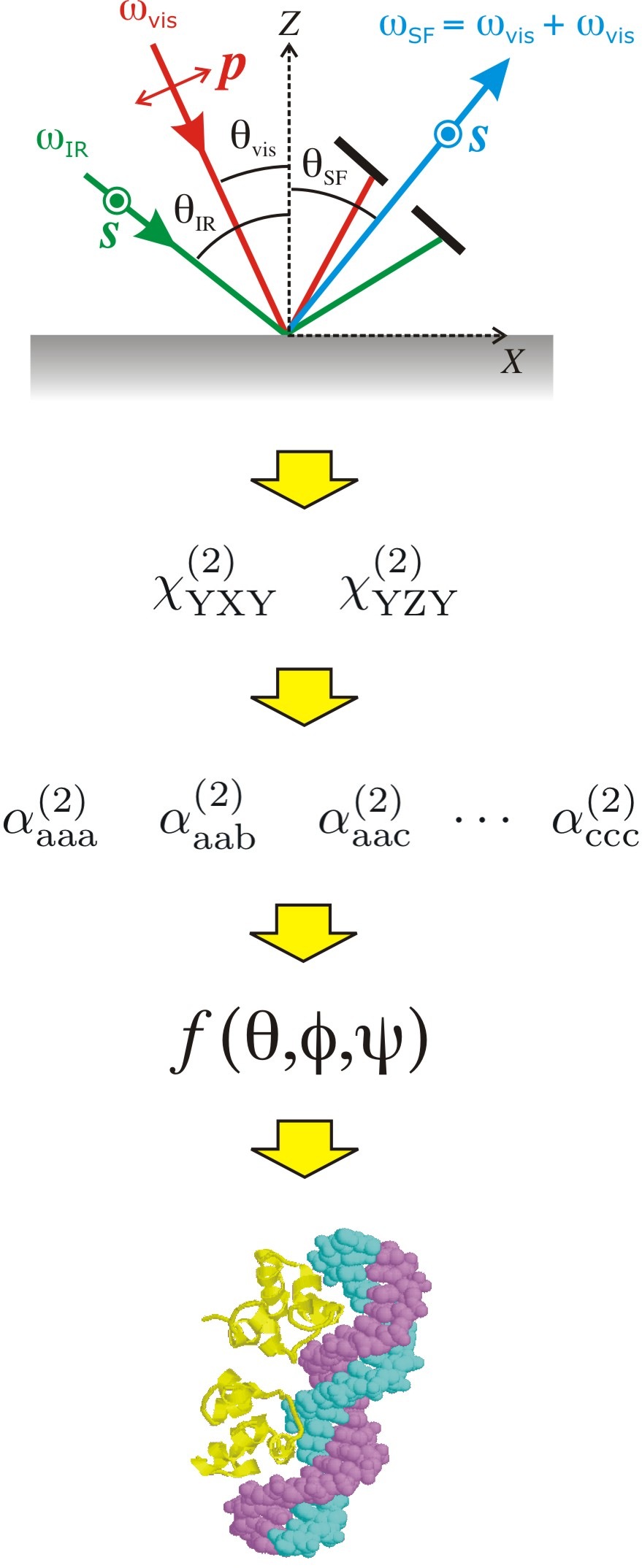

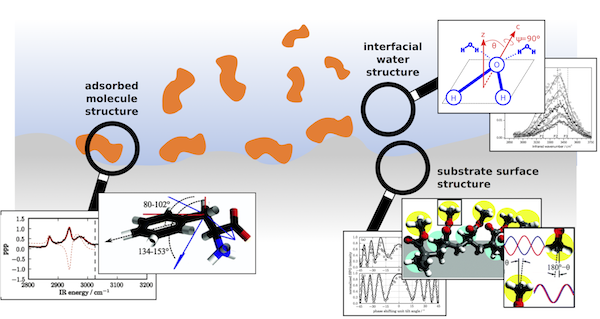
Research Area 1: The interplay between adsorbed molecule, interfacial solvent, and substrate surface structure
The conformation of proteins is well-recognized as being of paramount importance to their function in living systems and synthetic bio-devices. We are interested in the interaction of proteins with hydrophobic solid surfaces. Does the protein change its structure upon adsorption on the surface? What are the molecular-level chemical features of the protein and surface that are responsible for these changes? An increased understanding of these questions is vital to ensure the continued development of biomaterials such as implants and biosensors. Our group tackles these questions using a combination of experimental and theoretical approaches. In general, these questions require an investigation not only of the adsorbed molecule structure, but also of the surrounding solvent and surface characteristics of the solid surface.
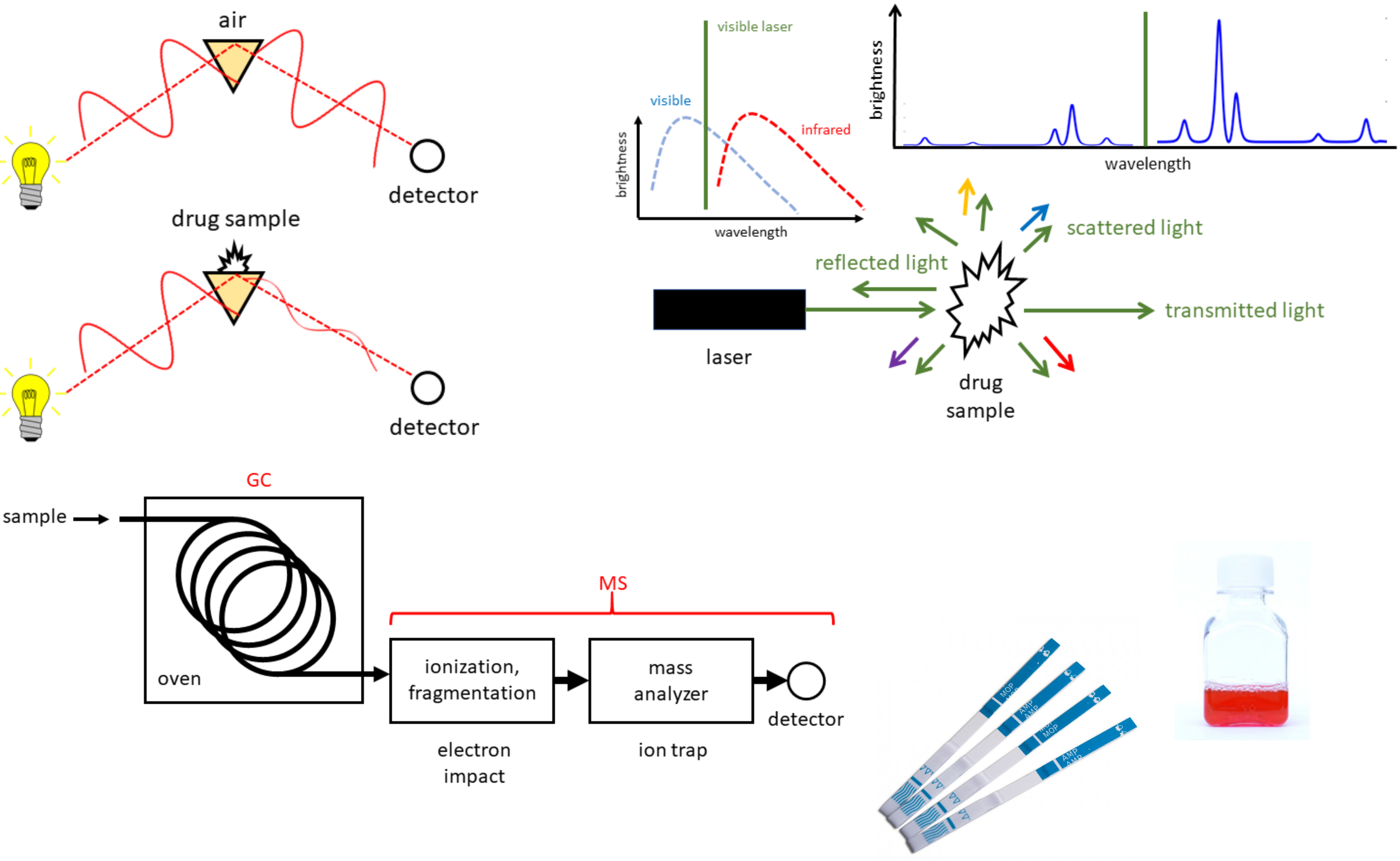
Research Area 2: Technologies for community-based drug checking
In response to the North America-wide opioid overdose crisis, we are working to develop new technologies and improve existing methods that enable people who use drugs to rapidly and accurately determine the composition and potentency of samples - challenges faced in the reality of an unregulated drug supply. Please refer to the substance.uvic.ca website for more information about our tools, hours, and service locations.
© DK Hore, Department of Chemistry, University of Victoria.