Neurophysiological Research:
Mechanisms of synaptic transmission and plasticity
Olfactory Bulb: Cellular and network analysis
Structural and synaptic defects in a mouse model of Rett syndrome
Postdoctoral and Grad student Positions Available 2022
Postdoctoral AND Grad student Positions available
Slugs for research
GARDEN SLUGS wanted for research


 1992 = before, 2008 = after, 2020 = way after...
You decide: Is science good for you? |
Dept. of Biology Box 1700 Stn CSC University of Victoria Victoria, British Columbia Canada V8W 2Y2 Voice-lab: (250) 472-5656 Voice-office: (250) 472-5657 FAX: (250) 721-7021 kdelaney@uvic.ca
|  Mitral cell apical dendrites filled with Oregon green BAPTA-1 dextran in an intact frog brain. This image is a maximum intensity projection of fifty optical sections obtained between 45 and 70 microns below the surface using 2-photon microscopy. FOV 90 microns wide. |
|---|
|
| 
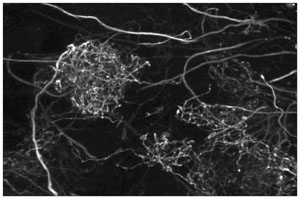
Olfactory bulb confocal maximum intensity projection image. 45 microns of depth of olfactory bulb is shown, coded
from red to purple. Neurons filled by local injection of dextran conjugated Alexa-488. Central neuron is a
granule cell. Spiny dendrites from out of frame granule cells as wells as smooth mitral cell dendrites and axons can
also be seen. Science = Art....
| |
|---|
Graduate Research in neuroscience at UVic Biology Dept.
Current trainees -- 2022 :
)
Research Staff:
List of Former Trainees
Research
Our laboratory is primarily interested in synaptic physiology which results in a wide diversity of projects
and opportunities for trainees with a variety of interests and experience. For all our work we combine electrophysiological
and optical techniques to monitor neural activity. These include whole cell patch clamp, sharp microelectrode and field potential recordings combined with
widefield CCD and 2-photon laser scanning imaging and photomultiplier based spot measurements, mostly using Ca2+ indicator
dyes or fluorescent proteins. We run a pair of two 2-photon microscopes off one laser, suitable for in vivo imaging and electrophysiology in small animals
(rats, mice and frogs) and for mammalian brain slice and isolated frog/turtle brains. Other than the lasers, these apparatus were
built from scratch using old car parts and stuff I "found" in the garbage cans at Bell labs two decades ago. A bit of an exaggeration
but they are definitely "home-made". A flexible 2-photon imaging program that coordinates acquisition of optical and electrophysiological
signals was written entirely by Dr. James Boyd working our lab using Igor (Wavemetrics) and C++. Many thanks are due to David Kleinfeld at UCSD
for some key electronics circuits and technical help along the way, and of course Herr Dr. Winfried Denk who taught me not to bring my
head down to tabletop level when the laser is on by cuffing me severely about the ears and yelling "nein dum...pf".
The interface between our CIHR and NSERC research programs develops from the concept that the processing of information by neural
networks can be modified by activity and modulator dependent changes in synaptic connections. We study basic properties of synaptic
transmission and apply this understanding to specific circuits and to investigate neural disorders.
Our NSERC supported research is directed towards understanding the cellular and biochemical basis for
activity-dependent and neuromodulator mediated enhancement of neurotransmitter release. Using microfluorometric imaging of fluorescent
Ca2+ indicator molecules or Ca2+-sensitive proteins we study the role of Ca2+-dependent processes in presynaptic terminals in modifying the probability
of transmitter release in response to action potential activity.
We study presynaptic calcium's role in synaptic transmission and plasticity at a variety of synapses including crayfish and frog neuromuscular
junctions, accessory olfactory bulb and vomeronasal organ Wanna look?.
Physiological measurements are combined with biophysical modeling studies to examine the consequences of molecular level
colocalization of channels and vesicles in presynaptic active zones for Ca2+ diffusion and transmitter release such as the work by
Vahid Shahrezaei (now faculty at Imperial College London)using numerical including
Monte-Carlo simulations. See also Vesicle
PDF1 , Vesicle PDF2
Further modeling and experimental work on the effect of reducing the calcium channel density for calcium channel cooperativity
at frog neuromuscular junction was undertaken using w-conotoxin to block channels
Vesicle PDF3 .
Past CIHR supported research includes projects integrating synaptic physiology and intrinsic electrophysiological properties of
neurons into a network level framework to understand the temporal spatial dynamics that underlie neural processing of sensory signals
by the olfactory bulb. For some of this work we have developed a novel in vitro nose-brain
preparation that allows brain slice type experiments to be performed in a system where normal patterns of afferent input can be activated
by application of odours to the nose. The work involves high speed imaging of Ca2+ and voltage sensitive dye signals from dendrites
and/or nerve terminals brain of frogs during stimulation of olfactory epithelia with odours. Complimentary electrophysiological studies
are used to relate the activity of individual neurons in the central nervous system (mitral cells and granule cells) to globally
distributed oscillatory activity in networks of millions of cells during odour-induced activity. Recordings from mitral and granule
cells during odour stimulation of the nose using the in vitro preparation are a significant part of this work. See Delaney
and Hall, 1996 and Hall and Delaney, 2002, Davison et al., 2004 for further details. Ben Hall is now at Lundbeck Pharmaceutical in Copenhagen.
Ian DAvison is on faculty in the Dept. of Biology at Boston University. Dr. Tibor Zelles of the Hungarian Academy, Institute for
Experimental Medicine in Budapest undertook studies on the active properties of dendrites
of granule cells using 2 photon imaging.
Dr. Jamie Johnston was supported by CIHR to study biophysical properties
and dendritic distribution of low-voltage activated, T-type calcium channels in mouse mitral cells using a combination of whole cell
electrophysiology and 2-photon calcium imaging. As of 2020 Dr. Johnston is a member of the research faculty at Leeds University.
Dr. Adam Fekete from Budapest continued eletrophysiological studies of olfactory bulb function and focused on the role of T-channels
in supporting transmitter release from mitral dendrites then moved to the Univ. of Toronto with Lu-Yang Wang
Rett Syndrome research: During the past 10 years we have been working Rett syndrome research using a loss of function mouse model
with a mutated non-functional Mecp2 gene (Jaenisch mouse). MeCP2 protein normally binds to methylated DNA acting primarily as a
transcription repressor to control many genes required for normal development. Loss of function in this gene is the cause of Rett
Syndrome a neurodevelopmental disorder leading to severe cognitive and other systemic dysfunctions primarily in female children. We have
crossed these mice with mice that express YFP in subsets of cortical neurons (Feng et al., 2000) to facilitate analysis of neuronal
morphologies associated with the Rett phenotype and as a tool to evaluate the success (or failure) of therapeutic interventions.
Current work (2022) funded by the International Rett Syndrome Foundation and CIHR investigates cell autonomous versus non-autonomous
effects of this X-linked mutation on synaptic transmission and development using immunohistochemistry
Rietveld et al., 2015/.
cell genotype deficits in nicotinic receptor sensitivity using a a Rett syndrome mouse model. More recent work
(2019) uses electrophysiology and optogenetics to study cell-genotype specific
effects on nicotinic cholinergic sensitivity in mutant versus wild-type neurons using slices (Azam Asgarihafshejani, now at U of T)
published in the journal Neuroscience. MeCP2 is X-linked and due to random X-inactivation female brains are a mosaic of mutant
and non-mutant neurons. To identify cell genotype in live slices we cross mice expressing an MeCP2-GFP fusion protein,
with Rett mice allows to visualize wild-type (green) versus mutant (not green) neurons for patching in female brains slices.
This work has been generously supported by the International Rett Syndrome Foundation, the Ontario Rett Syndrome Foundation
and currently by CIHR.
Physiological measurements are combined with biophysical modeling studies to examine the consequences of molecular level colocalization of channels and vesicles in presynaptic active zones for Ca2+ diffusion and transmitter release such as the work by Vahid Shahrezaei (now faculty at Imperial College London)using numerical including Monte-Carlo simulations. See also Vesicle PDF1 , Vesicle PDF2 Further modeling and experimental work on the effect of reducing the calcium channel density for calcium channel cooperativity at frog neuromuscular junction was undertaken using w-conotoxin to block channels Vesicle PDF3 .