

 |
 |
||||||
 |
|
| Keywords: inorganic chemistry, synthesis, stable radicals, redox-active ligands, coordination chemistry, indigo, catalysis, magnetism, materials. | |
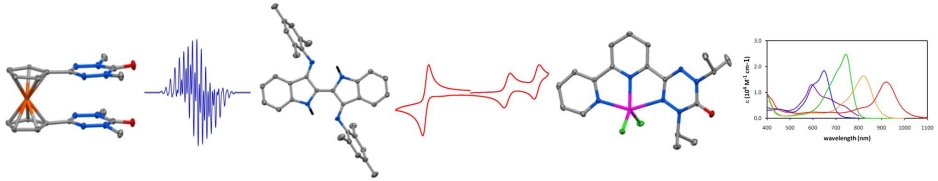
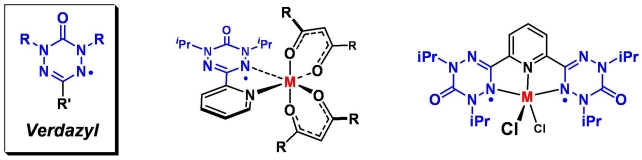
Overview The 'redox chemistry' (electron transfer, oxidative addition, reductive elimination) of transition metal complexes is one of their defining features. Normally these redox processes take place on the metal. However, certain kinds of ligands (redox-active ligands, also known as 'non-innocent' ligands) can actively participate in electron transfer. This characteristic has fascinated inorganic chemists for nearly 50 years and continues to do so because of the special challenges associated with elucidating the electronic structure of redox-active ligand complexes ("where are the valence electrons?"). In addition to these fundamental issues, a number of metalloenzyemes have redox-active ligand complexes as the active site (e.g. cytochrome P450, galactose oxidase). These naturally occuring systems have inspired recent worldwide efforts to develop new reagents and catalysts based on redox-active ligand complexes, in which the charge storage capabilities of redox-active ligands enables new reaction types. The vast majority of research on redox-active ligands focuses on a few common types such as the dithiolenes and dioxolenes. My group is interested in design, discovery, and development of new kinds of redox active ligands and their coordination complexes. Design: what makes a ligand redox-active, or stable with unpaired electrons? Discovery: What are the properties of non-traditional ligand and their metal complexes? Development: Can we make new metal complexes with “functional ligands” do interesting and useful things, create new opportunities, address contemporary scientific challenges? We’re principally a synthetic chemistry group, i.e. we make lots of molecules. Most of our target molecules require an array of studies to fully understand their electronic structure, which is a necessary first step in trying to ultimately do something useful with them. Techniques include many “conventional” characterization methods (NMR, IR, UVVis, MS) plus a suite of others (EPR, electrochemistry, magnetometry, photochemistry & photophysics, DFT calculations). Radical-based ligands Radicals are usually thought of as reactive intermediates – transient, unstable species. However there are exceptions, i.e. examples of molecules with unpaired electrons yet are stable enough to be made, handled, and stored like ordinary (closed shell) compounds. We’re interested in many different aspects of stable radical science ranging from the design of new kinds of radicals to magnetism to polymer synthesis. We’ve developed many new dimensions to the chemistry of verdazyls, heterocyclic radicals with outstanding stability but whose chemistry has not been well explored. We have been particularly active in developing the synthesis (publications 23,37) and magnetism of verdazyls (pubs 28,44,55,66) and their metal comlpexes (pubs 25, 26, 29, 30, 33, 35, 45, 48, 72). Our current interests continue to focus on the magnetochemistry of metal-verdazyls, and we're now actively engaged in the fundamental redox properties of verdazyls and their metal complexes (pubs 57, 67, 74) with a view to ultimately realizing new reagents and catalysts based on metal verdazyl complexes. |
|
 |
|
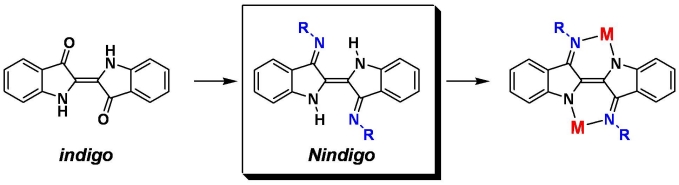
Organic dye-based ligands Indigo is one of the most famous molecules in the history of organic chemistry. Its brilliant blue colour (see below left), excellent envronmental stability, and ready availability from plant sources have made this species a popular textile pigment for thousands of years; indigo is produced today on the kiloton scale because it is the colorant in blue jeans. However, due to the very low solubility of indigo in common solvents the chemistry of this interesting molecule remains undeveloped, particularly its inorganic coordination chemistry. We’ve recently discovered a way to turn indigo into its corresponding diimine (‘Nindigo’) which renders the species much more soluble. The diimines are capable of binding one or two metals, are redox-active, and have very low-energy electronic absorption (see publications 71, 75). We’re exploring all sorts of multimetallic chemistry based on Nindigo and also developing similar metal chemistry of related dye molecules. |
|
 |
 |
Other projects the Hicks group has worked in other research areas, including:
|
|