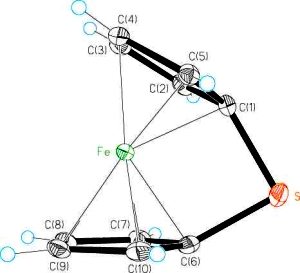
The development of catalytic reactions has revolutionised the synthesis of organic molecules and polymers. In contrast, catalysis is virtually unexplored as a route to molecular and macromolecular inorganic materials. Over the past decade our group has been at the forefront of the development of catalytic reactions with main group substrates. In particular, we have been involved in a broad expansion of this field in the area of dehydrogenation and dehydrocoupling processes that allow access to a wide range of catenated structures based on elements across the p-block. Such catalytic pathways using main group substrates represent an increasingly attractive and convenient alternative to traditional routes such as salt metathesis and reductive coupling reactions. Applications of this work involve the fields of hydrogen storage and transfer, functional inorganic polymers, and ceramic thin films.
Selected Publications:
- Catalysis in Service of Main Group Chemistry: a Versatile Approach to p-Block Molecules and Materials.
Leitao, E.M.; Jurca, T.; Manners, I.
Nat. Chem., 2013, 5, 857.
- Non-Metal-Catalyzed Heterodehydrocoupling of Phosphines and Hydrosilanes: Mechanistic Studies of B(C6F5)3-Mediated Formation of P-Si Bonds
Wu, L.; Chitnis, S. S.; Jiao, H.; Annibale, V. T.; Manners, I.
J. Am. Chem. Soc., 2017, 139, 16780.
- Addition of a Cyclophosphine to Nitriles: An Inorganic "Click" Reaction Featuring Protio-, Organo-, and Main Group Catalysis
Chitnis, S. S.; Sparkes, H. A.; Annibale, V. T.; Pridmore, N. E.; Oliver, A. M.; Manners, I.
Angew. Chem. Int. Ed., 2017, 56, 9536.
- Homo- and Heterodehydrocoupling of Phosphines Mediated by Alkali Metal Catalysts
Wu. L.; Annibale, V.; Jiao, H.; Brookfield, A.; Collison, D.; Manners, I.
Nat. Commun., 2019, 10, 2786.
- Metal-Free Dehydropolymerisation of Phosphine-Boranes using Cyclic (Alkyl)(Amino)Carbenes as Hydrogen Acceptors
Oldroyd, N.L.; Chitnis, S.S.; Annibale, V.T.; Arz, M.A.; Sparkes, H.A.; Manners, I.
Nat. Commun., 2019, 10, 1370.
- Trivalent Titanocene Alkyls and Hydrides as Well-Defined, Highly Active, and Broad Scope Precatalysts for Dehydropolymerization of Amine-Boranes
LaPierre, E.A.; Patrick, B.O.; Manners, I.
J. Am. Chem. Soc., 2019, 141, 20009.
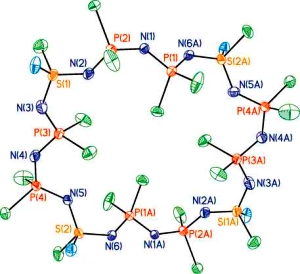
- Ring-Opening Polymerization of Cyclic Phosphonates: Access to Inorganic Polymers with a Pv-O Main Chain
Arz, M.A.; Annibale, V.T.; Kelly, N.L.; Hanna, J.V.; Manners, I.
J. Am. Chem. Soc., 2019, 141, 2894.
- Polyphosphinoborane Block Copolymer Synthesis Using Catalytic Reversible Chain-Transfer Dehydropolymerization
Race, J.J.; Heyam, A.; Wiebe, M.A.; Hernandez, J.D.G.; Ellis, C.E.; Lei, S.; Manners, I.; Weller, A.S.
Angewandte Chemie, 2023, 62, e202216106.
- Transition-Metal-Free Dehydropolymerization of Phosphine–Boranes at Ambient Temperature
Wiebe, M.A.; Kundu, S.; LaPierre, E.A.; Patrick, B.O.; Manners, I.
Chem. Eur. J., 2023, 29, e202202897.
- A Crystalline Monomeric Phosphaborene
LaPierre, E.A.; Patrick, B.O.; Manners, I.
J. Am. Chem. Soc., 2023, 145, 7107.
- Synthesis of a Carbene-Stabilized (Diphospha)aminyl Radical and Its One Electron Oxidation and Reduction to Nonclassical Nitrenium and Amide Species
LaPierre, E.A.; Wantanabe, L.; Patrick, B.O.; Rawson, J.M.; Tuononen, H.M.; Manners, I.
J. Am. Chem. Soc., 2023, 145, 9223.
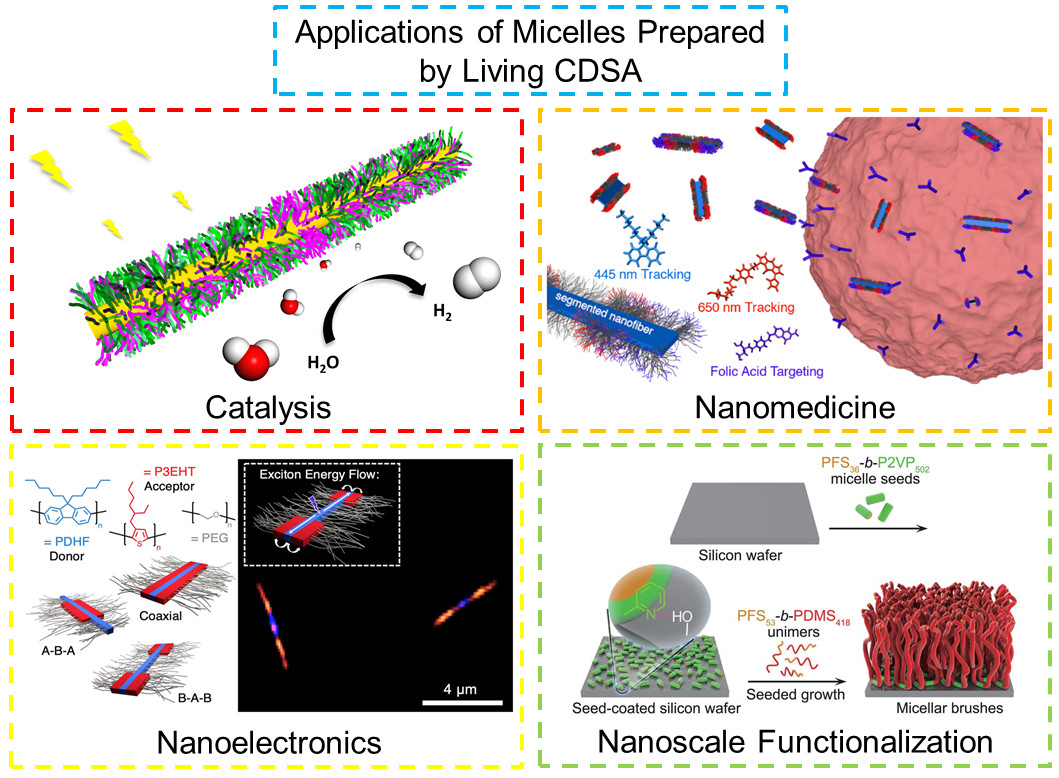
Our research group has been at the forefront of the development of "Living" Crystallization-Driven Self-Assembly (CDSA) of Block Copolymers and other building blocks such as planar π-stacking organic molecules and metallocycles to form well-defined colloidally-stable 1D and 2D materials with tunable dimensions, spatially controlled surface and core chemistries, and potential applications from information storage and nanoelectronics to biomedicine. A focus has been on polyferrocenylsilane block copolymers, which were developed in our group, but our more recent work has also involved block copolymers with crystallizable π-conjugated or biodegradable segments. Together with our collaborators, we are currently investigating the fundamentals of the fascinating living CDSA process and also a range of potential applications of the resulting phase-separated thin films and core-shell nanostructures (micelles) as nanowires, nanoscopic barcodes, self-assembled heterojunctions, catalysts, and as magnetic dot precursors and in drug/gene delivery.
Selected Publications:
- Multidimensional Hierarchical Self-Assembly of Amphiphilic Cylindrical Block Comicelles
Qiu, H.; Hudson, Z.M.; Winnik, M.A.; Manners, I.
Science, 2015, 347, 1329.
- Uniform patchy and hollow rectangular platelet micelles from crystallizable polymer blends
Qiu, H.; Gao, Y.; Boott, C.E.; Gould, O.E.C.; Harniman, R.L.; Miles, M.J.; Webb, S.E.D.; Winnik, M.A., Manners, I.
Science, 2016, 352, 697.
- Two dimensional assemblies from crystallizable homopolymers with charged termini
He, X.; Hsiao, M-S.; Boott, C.E.; Harniman, R.L.; Nazemi, A.; Li, X.; Winnik, M.A.; Manners, I.
Nat. Mater., 2017, 16, 481.
- Scalable and uniform 1D nanoparticles by synchronous polymerization, crystallization and self-assembly
Boott, C.E.; Gwyther, J.; Harniman, R.L.; Hayward, D.W.; Manners, I.
Nat. Chem., 2017, 9, 785.
- Uniform electroactive fiber-like micelle nanowires for organic electronics
Li, X.; Wolanin, P.J.; MacFarlane, L.R.; Harniman, R.L.; Qian, J.; Gould, O.E.C.; Dane, T.G.; Rudin, J.; Cryan, M.J.; Schmaltz, T.; Frauenrath, H.; Winnik, M.A.; Faul, C.F.J.; Manners, I.
Nat. Commun., 2017, 8, 15909.
- Long-range Exciton Transport in Conjugated Polymer Nanofibers Prepared by Seeded Growth
Jin, X.-H.; Price, M.B.; Finnegan, J.R.; Boott, C.E.; Richter, J.M.; Rao, A.; Menke, M.; Friend, R.H.; Whittell, G.R.; Manners, I.
Science, 2018, 360, 897.
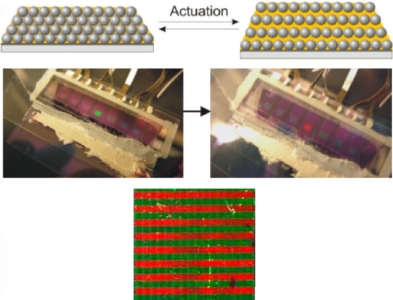
- Tailored Multifunctional Micellar Brushes via Crystallization-Driven Growth from a Surface
Cai, J.; Li, C.; Kong, N.; Lu, Y.; Lin, G.; Wang, X.; Yao, Y.; Manners, I.; Qiu, H.
Science, 2019, 366, 1095.
- Cellular Uptake and Targeting of Low Dispersity, Dual Emissive, Segmented Block Copolymer Nanofibers
Street, S.; He, Y.; Jin, X.; Hodgson, L.; Verkade, P.; Manners, I.
Chem. Sci., 2020, 11, 8394.
- Tailored Self-Assembled Photocatalytic Nanofibers for Visible-light Driven Hydrogen Production
Tian, J.; Zhang, Y.; He, Y.; Jin, X-H.; Pearce, S. Eloi, J-C.; Harniman, R.L.; Alibhai, D.; Ye, R.; Philiips, D.L.; Manners, I.
Nat. Chem., 2020, 12, 1150.
- Scalable and Uniform Length-Tunable Biodegradable Block Copolymer Nanofibers with a Polycarbonate Core via Living Polymerization-Induced Crystallization-Driven Self-assembly
Ellis, C.E.; Hernandez, J.D.G.; Manners, I.
J. Am. Chem. Soc., 2022, 144, 20525.
- Length-Controlled Nanofiber Micelleplexes as Efficient Nucleic Acid Delivery Vehicles
Street, S.T.G.; Chrenek, J.; Harniman, R.L.; Letwin, K.; Mantell, J.M.; Borucu, U.; Willerth, S.M.; Manners, I.
J. Am. Chem. Soc., 2022, 144, 19799.
- High Resolution Cryo-Electron Microscopy Structure of Block Copolymer Nanofibers with a Crystalline Core
Tian, J.; Xie, S.-H.; Borucu, U.; Lei, S.; Zhang, Y.; Manners, I.
Nat. Mater., 2023, 22, 786.
- Uniform Segmented Platelet Micelles with Compositionally Distinct and Selectively Degradable Cores
Tong, Z.; Xie, Y.; Arno, M.C.; Zhang, Y.; Manners, I.; O'Reilly, R.K.; Dove, A.P.
Nat. Chem., 2023, 15, 824.